Abstract
INTRODUCTION Post-transplant cyclophosphamide (PT-Cy) has become the standard graft-versus-host disease (GVHD) prophylaxis for haploidentical hematopoietic cell transplantation (HCT) and has been increasingly used in allogeneic HCT with HLA-matched donors. However, PT-Cy is associated with significant toxicities and organ damage, especially in elderly patients. While toxicities may be correlated with the total cyclophosphamide dose, few studies have evaluated the safety and efficacy of different PT-Cy doses. We aimed to compare the outcomes with a reduced PT-Cy total dose of 70 mg/kg to those with PT-Cy at 100 mg/kg in elderly patients and patients with cardiac comorbidities undergoing haploidentical HCT.
PATIENTS AND METHODS Inclusion criteria included: (1) age ≥ 65 years, or patients of any age with a history of cardiac events, (2) hematological malignancy, (3) haploidentical HCT, (4) peripheral blood stem cells, and (5) thiotepa-based conditioning regimen with antithymocyte globulin (ATG). Since 2015, our center policy has been PT-Cy at 100 mg/kg divided in 2 doses, to all patients. Starting from 2020, 40 patients have received PT-Cy at 35 mg/kg/day on day+3 and day+4 either because of older age or cardiac comorbidities.
RESULTS Fifty-eight patients met the inclusion criteria. Median age was 68 years (range, 15-76) and 60% of patients were male. Median HCT-specific comorbidity index (HCT-CI) score was 2 (range, 0-5). Patients were transplanted for acute myeloid leukemia (n=37, 64%), myelodysplastic syndrome (n=11, 19%), lymphoma (n=8, 14%), or myeloproliferative neoplasm (n=2, 3%). Disease risk index was intermediate in 22 (38%) patients and high or very high in 36 (62%). Disease status at transplantation was complete remission in 18 patients (31%). Conditioning regimens were reduced intensity thiotepa-based (thiotepa - busulfan - fludarabine) in 25 (43%) patients or Flamsa-like sequential (thiotepa - etoposide - cyclophosphamide, followed by fludarabine - busulfan) in 33 (57%). GVHD prophylaxis included cyclosporin, mycophenolate mofetil, and ATG in all patients. Thirty-three patients received PT-Cy at 70 mg/kg and 25 at 100 mg/kg. There was no significant difference between the 2 groups (PT-Cy at 70 mg/kg versus 100 mg/kg) in terms of age, gender, HCT-CI score, cardiovascular risk factors, type of hematological disease, disease risk index, disease status, and type of conditioning regimen. The median follow-up was 17 months (interquartile range [IQR], 13-20) and 59 months (IQR, 51-66) in patients receiving PT-Cy at 70 mg/kg and 100 mg/kg, respectively.
The cumulative incidences (CIs) of acute grade II-IV and grade III-IV GVHD were 18% and 0% with PT-Cy at 70 mg/kg compared to 17% and 5% with 100 mg/kg, respectively (p=0.94 for grade II-IV and p=0.87 for grade III-IV acute GVHD). The CI of chronic GVHD was 27% and 29% with PT-Cy at 70 mg/kg and 100 mg/kg, respectively (p=0.95). No significant difference was observed for moderate to severe chronic GVHD. Neutrophil recovery was significantly improved with 70 mg/kg compared to 100 mg/kg (median time of 16 versus 19 days, respectively, p=0.006). At days +30, +60, and +90 after HCT, 75%, 88% and 91% patients had a platelet count > 50 x 109/L with 70 mg/kg of PT-Cy, respectively, compared to 36%, 64%, and 64% with 100 mg/kg of PT-Cy, respectively (p=0.011). The CI of post-HCT bacteremia was 38% with PT-Cy at 70 mg/kg and 72% with 100 mg/kg (p=0.004). Other complications commonly associated with PT-Cy were also reduced with 70 mg/kg compared to 100 mg/kg of PT-Cy, such as BK-virus associated hemorrhagic cystitis (12% versus 28%) and left ventricular systolic dysfunction (9% versus 24%).
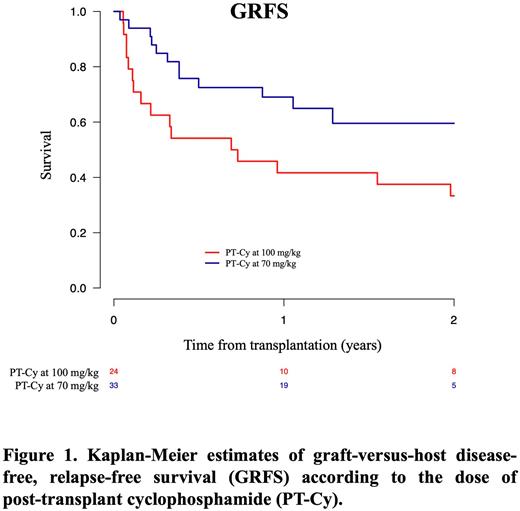
At 2 years, CIs of non-relapse mortality were 18% and 33% (p=0.13), progression free-survival 65% and 46% (p=0.09), overall survival 68% and 52% (p=0.31), and GVHD-free, relapse-free survival 60% and 33% (p=0.04) with 70 mg/kg and 100 mg/kg of PT-Cy, respectively.
CONCLUSIONS Reducing PT-Cy dose to 70 mg/kg is a safe and valid approach in elderly patients and patients with cardiac comorbidities underdoing haploidentical HCT with peripheral blood stem cells and low-dose ATG. Compared to 100 mg/kg, neutrophil and platelet recovery were improved, CIs of bacteremia, BK-virus associated hemorrhagic cystitis, and cardiac complications were reduced, the risk of acute and chronic GVHD was not increased, and GVHD-free, relapse-free survival was higher with PT-Cy at 70 mg/kg.
Disclosures
Dulery:Novartis: Honoraria; Bristol Myers squibb: Honoraria; Kite Gilead: Other: Scientific meeting subscription fees and travel support; Biotest: Honoraria; Takeda: Honoraria. Malard:Novartis: Honoraria; Takeda: Honoraria; Janssen: Honoraria; Biocodex: Honoraria; JAZZ pharmaceuticals: Honoraria; Astellas: Honoraria; Sanofi: Honoraria; Therakos/Mallinckrodt: Honoraria; Gilead: Honoraria; Celgene-BMS: Honoraria. Labopin:Jazz Pharmaceuticals: Honoraria. Mohty:Astellas: Honoraria; Amgen: Honoraria; Celgene: Honoraria; Adaptive Biotechnologies: Honoraria; Takeda: Honoraria; Bristol Myers Squibb: Honoraria; Novartis: Honoraria; Oncopeptides: Honoraria; Pfizer,: Honoraria; GSK: Honoraria; Jazz Pharmaceuticals: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Gilead: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal